Session: ePoster: Colon and Colorectal Cancer 20 (Tue8)
EP941 - Comparative Survival Analysis of Trifluridine/Tipiracil + Bevacizumab Versus Regorafenib in Metastatic Colorectal Cancer: Insights from a Real-World TriNetX Database Study
Tuesday, May 13, 2025
8:10 AM - 8:15 AM PT
Location: Monitor 6, Sails Pavilion, Upper Level
.jpg)
CHIH AN LIN, MD, Colorectal Surgeon
Attending physician
Taichung Veterans General Hospital, Department of Colorectal Surgery
TAICHUNG, Taichung, Taiwan (Republic of China)All of the relevant financial relationships listed below have been mitigated.
This individual has no financial relationships with ineligible companies..jpg)
Chang Lin Lin
Attending physician
colorectal surgery
Taichung, Taichung, Taiwan (Republic of China)All of the relevant financial relationships listed below have been mitigated.
This individual has no financial relationships with ineligible companies.
ePoster Presenter(s)
Senior Author(s)
All of the relevant financial relationships listed below have been mitigated.
CHIH AN LIN, MD, Colorectal Surgeon: This individual has no financial relationships with ineligible companies.
Chang Lin Lin: This individual has no financial relationships with ineligible companies.
Purpose/Background: Colorectal cancer is the third most commonly diagnosed cancer worldwide and the second leading cause of cancer-related deaths. For metastatic colorectal cancer (mCRC), the five-year survival rate is approximately 15%. Trifluridine/tipiracil (FTD/TPI, also known as TAS-102) and regorafenib are the standard third-line treatments for chemotherapy-refractory mCRC. The SUNLIGHT study demonstrated that adding bevacizumab to FTD/TPI significantly improved overall survival (OS). This study evaluates real-world survival outcomes of FTD/TPI + bevacizumab compared to FTD/TPI alone and regorafenib using the TriNetX global research network.
Methods/Interventions: This retrospective cohort study utilized the TriNetX global research network, which includes data from 127 healthcare organizations across multiple countries and over 300 million patients. Patients with mCRC were identified using ICD-10 codes for colon, rectosigmoid junction, and rectal cancers from 2015 to 2023. The study population was categorized into three cohorts: FTD/TPI alone, FTD/TPI + bevacizumab, and regorafenib. Propensity score matching was used to balance baseline characteristics such as age, sex, ECOG performance status, and comorbidities. The primary outcome was OS, measured from the start of third-line therapy until death or last follow-up. Secondary outcomes included PFS and treatment-related adverse events. Survival analyses were performed using Kaplan-Meier curves and Cox proportional hazards models, with HR and 95% CI reported for comparisons.
Results/Outcomes: A total of 5,213 patients met the initial inclusion criteria: 501 in the FTD/TPI + bevacizumab group, 3,937 in the FTD/TPI group, and 2,775 in the regorafenib group. After propensity score matching, two direct comparisons were conducted to assess the survival impact of FTD/TPI + bevacizumab. In the first comparison, FTD/TPI + bevacizumab was evaluated against FTD/TPI alone, resulting in a matched cohort of 499 patients in each group. The median OS was significantly longer in the FTD/TPI + bevacizumab group compared to the FTD/TPI monotherapy group (12.7 months vs. 9.0 months, HR = 0.782, 95% CI = 0.667–0.916, p < 0.002). The Kaplan-Meier survival curves indicated a clear separation, favoring the FTD/TPI + bevacizumab combination in terms of OS.
The second comparison analyzed FTD/TPI + bevacizumab against regorafenib, resulting in a matched cohort of 499 patients in each group. Similarly, the median OS was significantly longer in the FTD/TPI + bevacizumab group compared to the regorafenib group (12.5 months vs. 9.4 months, HR = 0.814, 95% CI = 0.694–0.954, p = 0.011). The Kaplan-Meier survival curves demonstrated a significant survival advantage in favor of the FTD/TPI + bevacizumab group, suggesting a more favorable outcome compared to single-agent regorafenib.
Conclusion/Discussion: This real-world analysis using the TriNetX network supports the SUNLIGHT study findings, showing that FTD/TPI with bevacizumab provides superior survival benefits over FTD/TPI alone and regorafenib in chemotherapy-refractory mCRC patients. These results confirm FTD/TPI + bevacizumab as a strong third-line option. This study emphasizes the need to consider FTD/TPI + bevacizumab as a preferred strategy for mCRC patients who have exhausted standard chemotherapy. Further prospective studies are needed to validate these findings and refine treatment strategies.
Kaplan-Meier Survival Curves Comparing Overall Survival in mCRC Patients Treated with FTD/TPI + Bevacizumab vs. Regorafenib
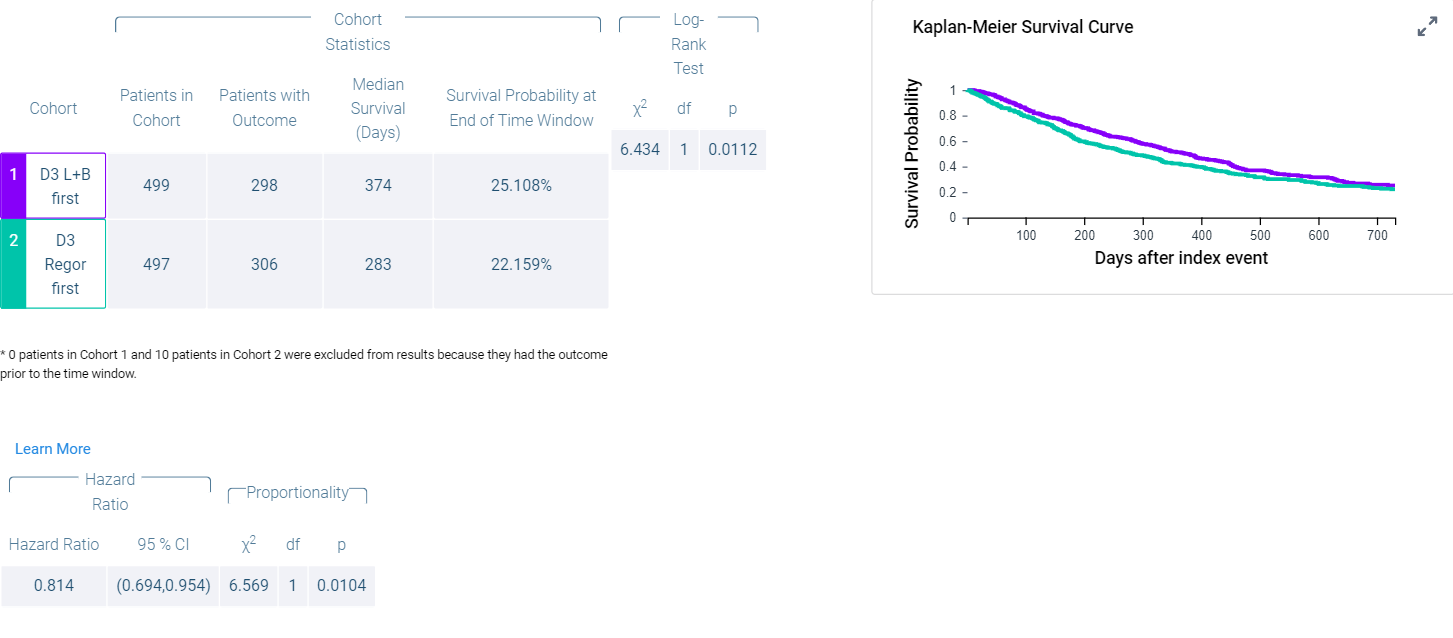 Kaplan-Meier survival curves and cohort statistics for patients receiving FTD/TPI + bevacizumab (Cohort 1) versus regorafenib (Cohort 2) as third-line treatments for chemotherapy-refractory metastatic colorectal cancer (mCRC). The median overall survival (OS) was 374 days (12.5 months) for the FTD/TPI + bevacizumab group and 283 days (9.4 months) for the regorafenib group. The hazard ratio (HR) was 0.814 (95% CI: 0.694–0.954, p = 0.0112), indicating a significant survival benefit for the FTD/TPI + bevacizumab group. Log-rank test results further confirmed the statistical significance of the survival difference (χ² = 6.434, p = 0.0112).
Kaplan-Meier survival curves and cohort statistics for patients receiving FTD/TPI + bevacizumab (Cohort 1) versus regorafenib (Cohort 2) as third-line treatments for chemotherapy-refractory metastatic colorectal cancer (mCRC). The median overall survival (OS) was 374 days (12.5 months) for the FTD/TPI + bevacizumab group and 283 days (9.4 months) for the regorafenib group. The hazard ratio (HR) was 0.814 (95% CI: 0.694–0.954, p = 0.0112), indicating a significant survival benefit for the FTD/TPI + bevacizumab group. Log-rank test results further confirmed the statistical significance of the survival difference (χ² = 6.434, p = 0.0112).
Kaplan-Meier Survival Curves Comparing Overall Survival of mCRC Patients Treated with FTD/TPI + Bevacizumab vs. FTD/TPI Monotherapy
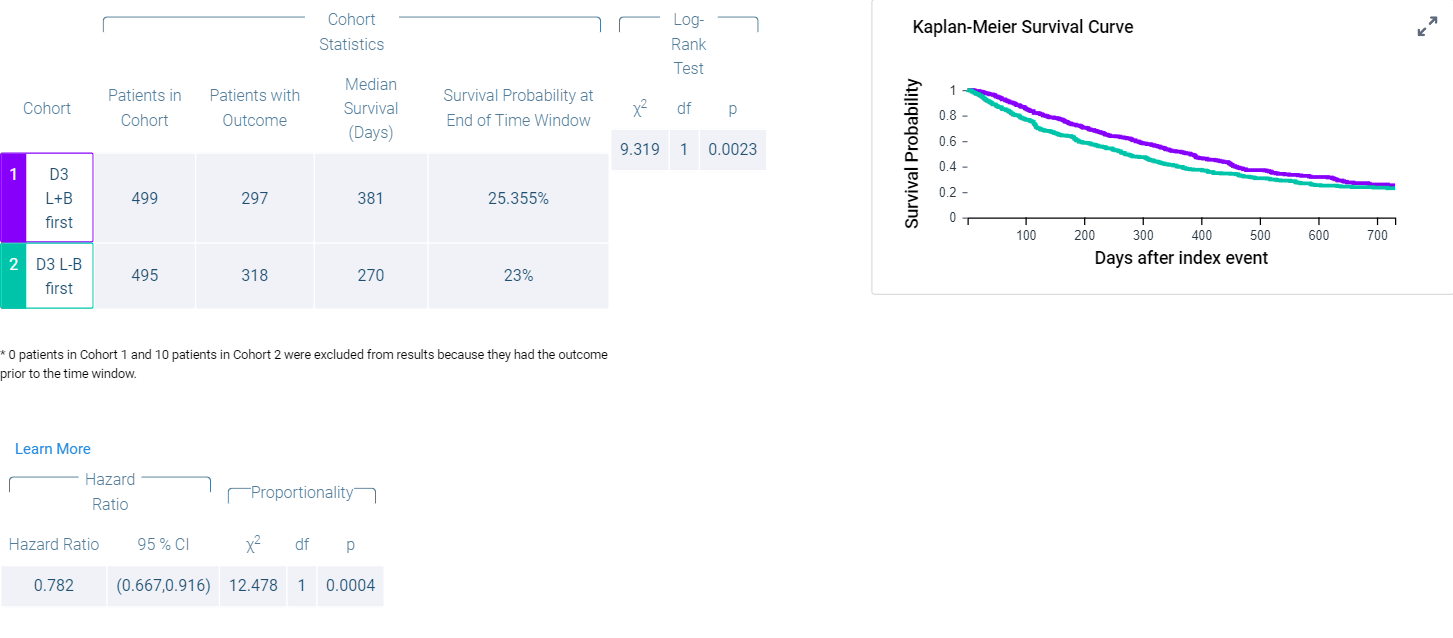 aplan-Meier survival curves and cohort statistics comparing overall survival (OS) of metastatic colorectal cancer (mCRC) patients receiving FTD/TPI + bevacizumab (Cohort 1, purple line) versus FTD/TPI monotherapy (Cohort 2, blue line) as third-line treatments. Cohort 1 had a median OS of 381 days (12.7 months), while Cohort 2 had a median OS of 270 days (9.0 months). The hazard ratio (HR) was 0.782 (95% CI: 0.667–0.916, p = 0.0004), indicating a significant survival advantage for the FTD/TPI + bevacizumab group. The log-rank test results confirmed a significant difference in survival between the two groups (χ² = 12.478, p = 0.0004).
aplan-Meier survival curves and cohort statistics comparing overall survival (OS) of metastatic colorectal cancer (mCRC) patients receiving FTD/TPI + bevacizumab (Cohort 1, purple line) versus FTD/TPI monotherapy (Cohort 2, blue line) as third-line treatments. Cohort 1 had a median OS of 381 days (12.7 months), while Cohort 2 had a median OS of 270 days (9.0 months). The hazard ratio (HR) was 0.782 (95% CI: 0.667–0.916, p = 0.0004), indicating a significant survival advantage for the FTD/TPI + bevacizumab group. The log-rank test results confirmed a significant difference in survival between the two groups (χ² = 12.478, p = 0.0004).
Methods/Interventions: This retrospective cohort study utilized the TriNetX global research network, which includes data from 127 healthcare organizations across multiple countries and over 300 million patients. Patients with mCRC were identified using ICD-10 codes for colon, rectosigmoid junction, and rectal cancers from 2015 to 2023. The study population was categorized into three cohorts: FTD/TPI alone, FTD/TPI + bevacizumab, and regorafenib. Propensity score matching was used to balance baseline characteristics such as age, sex, ECOG performance status, and comorbidities. The primary outcome was OS, measured from the start of third-line therapy until death or last follow-up. Secondary outcomes included PFS and treatment-related adverse events. Survival analyses were performed using Kaplan-Meier curves and Cox proportional hazards models, with HR and 95% CI reported for comparisons.
Results/Outcomes: A total of 5,213 patients met the initial inclusion criteria: 501 in the FTD/TPI + bevacizumab group, 3,937 in the FTD/TPI group, and 2,775 in the regorafenib group. After propensity score matching, two direct comparisons were conducted to assess the survival impact of FTD/TPI + bevacizumab. In the first comparison, FTD/TPI + bevacizumab was evaluated against FTD/TPI alone, resulting in a matched cohort of 499 patients in each group. The median OS was significantly longer in the FTD/TPI + bevacizumab group compared to the FTD/TPI monotherapy group (12.7 months vs. 9.0 months, HR = 0.782, 95% CI = 0.667–0.916, p < 0.002). The Kaplan-Meier survival curves indicated a clear separation, favoring the FTD/TPI + bevacizumab combination in terms of OS.
The second comparison analyzed FTD/TPI + bevacizumab against regorafenib, resulting in a matched cohort of 499 patients in each group. Similarly, the median OS was significantly longer in the FTD/TPI + bevacizumab group compared to the regorafenib group (12.5 months vs. 9.4 months, HR = 0.814, 95% CI = 0.694–0.954, p = 0.011). The Kaplan-Meier survival curves demonstrated a significant survival advantage in favor of the FTD/TPI + bevacizumab group, suggesting a more favorable outcome compared to single-agent regorafenib.
Conclusion/Discussion: This real-world analysis using the TriNetX network supports the SUNLIGHT study findings, showing that FTD/TPI with bevacizumab provides superior survival benefits over FTD/TPI alone and regorafenib in chemotherapy-refractory mCRC patients. These results confirm FTD/TPI + bevacizumab as a strong third-line option. This study emphasizes the need to consider FTD/TPI + bevacizumab as a preferred strategy for mCRC patients who have exhausted standard chemotherapy. Further prospective studies are needed to validate these findings and refine treatment strategies.
Kaplan-Meier Survival Curves Comparing Overall Survival in mCRC Patients Treated with FTD/TPI + Bevacizumab vs. Regorafenib
 Kaplan-Meier survival curves and cohort statistics for patients receiving FTD/TPI + bevacizumab (Cohort 1) versus regorafenib (Cohort 2) as third-line treatments for chemotherapy-refractory metastatic colorectal cancer (mCRC). The median overall survival (OS) was 374 days (12.5 months) for the FTD/TPI + bevacizumab group and 283 days (9.4 months) for the regorafenib group. The hazard ratio (HR) was 0.814 (95% CI: 0.694–0.954, p = 0.0112), indicating a significant survival benefit for the FTD/TPI + bevacizumab group. Log-rank test results further confirmed the statistical significance of the survival difference (χ² = 6.434, p = 0.0112).
Kaplan-Meier survival curves and cohort statistics for patients receiving FTD/TPI + bevacizumab (Cohort 1) versus regorafenib (Cohort 2) as third-line treatments for chemotherapy-refractory metastatic colorectal cancer (mCRC). The median overall survival (OS) was 374 days (12.5 months) for the FTD/TPI + bevacizumab group and 283 days (9.4 months) for the regorafenib group. The hazard ratio (HR) was 0.814 (95% CI: 0.694–0.954, p = 0.0112), indicating a significant survival benefit for the FTD/TPI + bevacizumab group. Log-rank test results further confirmed the statistical significance of the survival difference (χ² = 6.434, p = 0.0112).Kaplan-Meier Survival Curves Comparing Overall Survival of mCRC Patients Treated with FTD/TPI + Bevacizumab vs. FTD/TPI Monotherapy
 aplan-Meier survival curves and cohort statistics comparing overall survival (OS) of metastatic colorectal cancer (mCRC) patients receiving FTD/TPI + bevacizumab (Cohort 1, purple line) versus FTD/TPI monotherapy (Cohort 2, blue line) as third-line treatments. Cohort 1 had a median OS of 381 days (12.7 months), while Cohort 2 had a median OS of 270 days (9.0 months). The hazard ratio (HR) was 0.782 (95% CI: 0.667–0.916, p = 0.0004), indicating a significant survival advantage for the FTD/TPI + bevacizumab group. The log-rank test results confirmed a significant difference in survival between the two groups (χ² = 12.478, p = 0.0004).
aplan-Meier survival curves and cohort statistics comparing overall survival (OS) of metastatic colorectal cancer (mCRC) patients receiving FTD/TPI + bevacizumab (Cohort 1, purple line) versus FTD/TPI monotherapy (Cohort 2, blue line) as third-line treatments. Cohort 1 had a median OS of 381 days (12.7 months), while Cohort 2 had a median OS of 270 days (9.0 months). The hazard ratio (HR) was 0.782 (95% CI: 0.667–0.916, p = 0.0004), indicating a significant survival advantage for the FTD/TPI + bevacizumab group. The log-rank test results confirmed a significant difference in survival between the two groups (χ² = 12.478, p = 0.0004).
